Generation of Solar Cell
Energy
Energy is the capacity to do work and is required for life processes. An energy resource is something that can produce heat, power life, move objects, or produce electricity. Matter that stores energy is called fuel. Human energy consumption has grown steadily .throughout human history. Early humans had modest energy requirements, mostly food and fuel for fires to cook and keep warm. In today's society, humans consume as much as 110 times as much energy per person as early humans. Most of the energy we use today comes from fossil fuels (stored solar energy). But fossils fuels have a disadvantage in that they are non-renewable on a human time scale, and cause other potentially harmful effects on the environment. In any event, the exploitation of all energy sources (with the possible exception of direct solar energy used for heating), ultimately rely on materials on planet Earth.
First questions we want to answer in this discussion:
- What sources of Energy are available?
- How do the energy sources rely on resources available on Earth?
- Which energy sources are renewable on a human time scale?
- Since fossil fuels (oil, natural gas, coal) are our main source of energy, how are they formed, how do we find them and exploit them?
- What is the future for our energy needs?
Energy Sources
There are 5 fundamental sources of energy:
- Nuclear fusion in the Sun (solar energy)
- Gravity generated by the Earth & Moon.
- Nuclear fission reactions.
- Energy in the interior of the Earth.
- Energy stored in chemical bonds.
Basically, in this post, I am going to talk about Solar Energy.
Solar Energy
Solar Energy arrives from the Sun by electromagnetic radiation. It can be used directly for heat and converted to electricity for other uses. It is a nearly unlimited source, it is renewable, and largely, non-polluting.
Solar cell
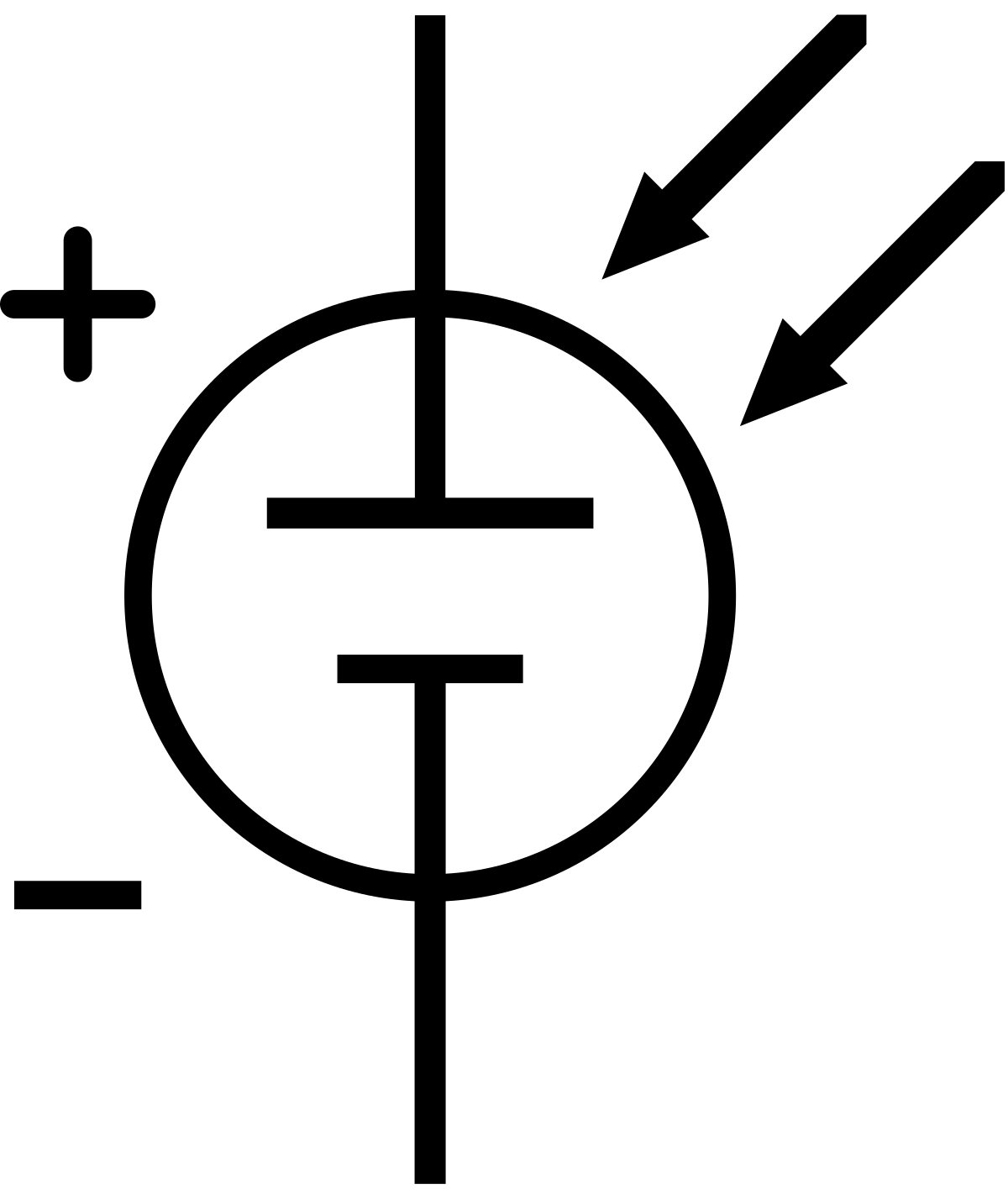
A solar cell, or photovoltaic cell, is an electrical device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon. It is a form of photoelectric cell, defined as a device whose electrical characteristics, such as current, voltage, or resistance, vary when exposed to light. Individual solar cell devices can be combined to form modules, otherwise known as solar panels. In basic terms, a single junction silicon solar cell can produce a maximum open-circuit voltage of approximately 0.5 to 0.6 volts.
Solar cells are described as being photovoltaic, irrespective of whether the source is sunlight or artificial light. They are used as a photodetector (for example infrared detectors), detecting light or other electromagnetic radiation near the visible range, or measuring light intensity.
The operation of a photovoltaic (PV) cell requires three basic attributes:
- The absorption of light, generating either electron-hole pairs or excitons.
- The separation of charge carriers of opposite types.
- The separate extraction of those carriers to an external circuit.
Brief History of Solar Cell
The photovoltaic effect was experimentally demonstrated first by French physicist Edmond Becquerel. In 1839, at age 19, he built the world's first photovoltaic cell in his father's laboratory.
Willoughby Smith first described the "Effect of Light on Selenium during the passage of an Electric Current" in a 20 February 1873 issue of Nature. In 1883 Charles Fritts built the first solid-state photovoltaic cell by coating the semiconductor selenium with a thin layer of gold to form the junctions; the device was only around 1% efficient. Other milestones include:
- 1888 – Russian physicist Aleksandr Stoletov built the first cell based on the outer photoelectric effect discovered by Heinrich Hertz in 1887.
- 1905 – Albert Einstein proposed a new quantum theory of light and explained the photoelectric effect in a landmark paper, for which he received the Nobel Prize in Physics in 1921.
- 1941 – Vadim Lashkaryov discovered p-n-junctions in Cu2O and Ag2S protocells.
- 1946 – Russell Ohl patented the modern junction semiconductor solar cell,[8] while working on the series of advances that would lead to the transistor.
- 1954 – the first practical photovoltaic cell was publicly demonstrated at Bell Laboratories.[9] The inventors were Calvin Souther Fuller, Daryl Chapin, and Gerald Pearson.
- 1958 – solar cells gained prominence with their incorporation onto the Vanguard I satellite.
Adjusting for inflation, it cost $96 per watt for a solar module in the mid-1970s. Process improvements and a very large boost in production have brought that figure down 99%, to 68¢ per watt in 2016, according to data from Bloomberg New Energy Finance.
During the 1990s, polysilicon ("poly") cells became increasingly popular. These cells offer less efficiency than their monosilicon ("mono") counterparts, but they are grown in large vats that reduce cost. By the mid-2000s, poly was dominant in the low-cost panel market, but more recently the mono returned to widespread use.
Solar Energy Theory
The solar cell works in several steps:
- Photons in sunlight hit the solar panel and are absorbed by semiconducting materials, such as silicon.
- Electrons are excited from their current molecular/atomic orbital. Once excited an electron can either dissipate the energy as heat and return to its orbital or travel through the cell until it reaches an electrode. Current flows through the material to cancel the potential and this electricity is captured. The chemical bonds of the material are vital for this process to work, and usually, silicon is used in two layers, one layer being doped with boron, the other phosphorus. These layers have different chemical electric charges and subsequently both drive and direct the current of electrons.
- An array of solar cells converts solar energy into a usable amount of direct current (DC) electricity.
- An inverter can convert the power to alternating current (AC).
Efficiency
Solar cell efficiency may be broken down into reflectance efficiency, thermodynamic efficiency, charge carrier separation efficiency and conductive efficiency. The overall efficiency is the product of these individual metrics.
The power conversion efficiency of a solar cell is a parameter which is defined by the fraction of incident power converted into electricity.
Single p–n junction crystalline silicon devices are now approaching the theoretical limiting power efficiency of 33.16%,[41] noted as the Shockley–Queisser limit in 1961. In the extreme, with an infinite number of layers, the corresponding limit is 86% using concentrated sunlight.[42]
In 2014, three companies broke the record of 25.6% for a silicon solar cell. Panasonic's was the most efficient.
In 2015, a 4-junction GaInP/GaAs//GaInAsP/GaInAs solar cell achieved a new laboratory record efficiency of 46.1 percent (concentration ratio of sunlight = 312) in a French-German collaboration between the Fraunhofer Institute for Solar Energy Systems (Fraunhofer ISE), CEA-LETI and SOITEC.
Materials
Solar cells are typically named after the semiconducting material they are made of. These materials must have certain characteristics in order to absorb sunlight. Some cells are designed to handle sunlight that reaches the Earth's surface, while others are optimized for use in space. Solar cells can be made of only one single layer of light-absorbing material (single-junction) or use multiple physical configurations (multi-junctions) to take advantage of various absorption and charge separation mechanisms.
Solar cells can be classified into first, second and third generation cells. The first generation cells—also called conventional, traditional or wafer-based cells—are made of crystalline silicon, the commercially predominant PV technology, that includes materials such as polysilicon and monocrystalline silicon. Second generation cells are thin film solar cells, that include amorphous silicon, CdTe and CIGS cells and are commercially significant in utility-scale photovoltaic power stations, building integrated photovoltaics or in small stand-alone power system. The third generation of solar cells includes a number of thin-film technologies often described as emerging photovoltaics—most of them have not yet been commercially applied and are still in the research or development phase. Many use organic materials, often organometallic compounds as well as inorganic substances. Despite the fact that their efficiencies had been low and the stability of the absorber material was often too short for commercial applications, there is a lot of research invested into these technologies as they promise to achieve the goal of producing low-cost, high-efficiency solar cells.
- Crystalline silicon
- Monocrystalline silicon
- Epitaxial silicon
- Polycrystalline silicon or multicrystalline silicon
- Ribbon silicon
- Mono-like-multi silicon (MLM)
- Thin film
- Cadmium Telluride
- Copper indium gallium selenide
- Silicon thin film
- Gallium arsenide thin film
- Multijunction cells
- GaInP/Si dual-junction solar cells
- Perovskite solar cells - Perovskite solar cells are solar cells that include a perovskite-structured material as the active layer.
- Bifacial solar cells - With a transparent rear side, bifacial solar cells can absorb light from both the front and rear sides. Hence, they can produce more electricity than conventional monofacial solar cells.
- Intermediate Band - Intermediate band photovoltaics in solar cell research provides methods for exceeding the Shockley–Queisser limit on the efficiency of a cell. It introduces an intermediate band (IB) energy level in between the valence and conduction bands. Theoretically, introducing an IB allows two photons with energy less than the bandgap to excite an electron from the valence band to the conduction band. This increases the induced photocurrent and thereby efficiency.
- Liquid ink - In 2014, researchers at California NanoSystems Institute discovered using kesterite and perovskite improved electric power conversion efficiency for solar cells.
- Upconversion and downconversion
- Light-absorbing dye - Dye-sensitized solar cells (DSSCs) are made of low-cost materials and do not need elaborate manufacturing equipment so they can be made in a DIY fashion.
- Quantum dots - Quantum dot solar cells (QDSCs) are based on the Gratzel cell or dye-sensitized solar cell architecture, but employ low bandgap semiconductor nanoparticles, fabricated with crystallite sizes small enough to form quantum dots (such as CdS, CdSe, Sb
2S
3, PbS, etc.), instead of organic or organometallic dyes as light absorbers. - Upconversion and downconversion - Photon upconversion is the process of using two low-energy (e.g., infrared) photons to produce one higher energy photon; downconversion is the process of using one high energy photon (e.g.,, ultraviolet) to produce two lower energy photons. Either of these techniques could be used to produce higher efficiency solar cells by allowing solar photons to be more efficiently used. The difficulty, however, is that the conversion efficiency of existing phosphors exhibiting up- or down-conversion is low, and is typically narrow band.
- Organic/polymer solar cells - Organic solar cells and polymer solar cells are built from thin films (typically 100 nm) of organic semiconductors.
- Adaptive cells - Adaptive cells change their absorption/reflection characteristics depending to respond to environmental conditions. An adaptive material responds to the intensity and angle of the incident light. At the part of the cell where the light is most intense, the cell surface changes from reflective to adaptive, allowing the light to penetrate the cell. The other parts of the cell remain reflective increasing the retention of the absorbed light within the cell.
- Surface texturing - Surface texturing is one of the techniques used to reduce optical losses to maximize light absorbed.
- Encapsulation - Solar cells are commonly encapsulated in transparent polymeric resin to protect the delicate solar cell regions for coming into contact with moisture, dirt, ice, and other conditions expected either during operation or when used outdoors. The encapsulants are commonly made from polyvinyl acetate or glass.
Tin(II) sulfide is an interesting potential candidate for next-generation thin film solar cells. Currently, both Cadmium Telluride and CIGS (Copper Indium Gallium Sulfide) are used as p-type absorber layers, but they are formulated from toxic, scarce constituents.[8] Tin(II) sulfide, by contrast, is formed from cheap, earth-abundant elements, and is nontoxic. This material also has a high optical absorption coefficient, p-type conductivity, and a mid-range direct band gap of 1.3-1.4 eV, required electronic properties for this type of absorber layer.[9] Based on the detailed balance calculation using the material bandgap, the power conversion efficiency of a solar cell utilizing a tin(II) sulfide absorber layer could be as high as 32%, which is comparable to crystalline silicon.[10] Finally, Tin(II) sulfide is stable in both alkaline and acidic conditions.[11] All aforementioned characteristics suggest tin(II) sulfide as an interesting material to be used as a solar cell absorber layer.
At present, tin(II) sulfide thin films for use in photovoltaic cells are still in the research phase of development with power conversion efficiencies currently less than 5%.[12] Barriers for use include a low open circuit voltage and an inability to realize many of the above properties due to challenges in fabrication, but tin(II) sulfide still remains a promising material if these technical challenges are overcome.
The Research and Document links are in this Google Docs File.














Comments
Post a Comment